4Dermis (Czech Health Research Council)
Cell-mediated multilayered 4th generation biomimetic scaffold for single-step total skin substitute:
from laboratory to clinical application

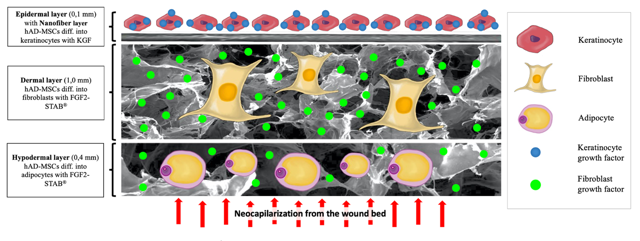
The aims of the project lie in the preparation and preclinical testing of a unique multilayer biomaterial based on natural resorbable biopolymers seeded with differentiated cell populations of xenogeneic mesenchymal stromal cells (MSCs) in a combination with stable growth factors. A partial goal will be the preparation of individual porous biopolymer layers containing collagen with bioactive polysaccharides and growth factors using nanotechnologies. The obtained materials will be tested for biomechanics,biodegradation and cytotoxicity. The methodology for the collection of human adipose-derived MSCs (hAD-MSCs) will also be validated, their cultivation and in the case of final preparation also cells differentiation directly in the prepared biomaterial within the laboratory operating in good manufacturing practice under defined conditions required for drug production. The individual layers of biomaterials containing growth factors will be connected to the resulting total skin substitute and in-vivo tested on an animal model. Because xenogeneic (human) AD-MSCs will be applied in the phase of full differentiation, it will be necessary to introduce an immunosuppressed pig model as well. The experiments will be evaluated by a complex histological analysis including classical staining, immunohistochemical staining and PCR, in order to find the most suitable combination of biomaterial layer composition As part of the complex regeneration of lost tissue, a fourth-generation scaffold containing different growth factors in individual layers will be used, as well as hAD-MSCs differentiated into different cell populations. The final production of biocompatible material combining cells and layered scaffolds will take place in special laboratories (clean rooms of class A / B / C) operating in the mode of Good Manufacturing Practice (GMP), which meet all the requirements required by the State Institute for Drug Control to produce Advanced Therapy Medicinal Product (ATMP).
Co-applicant: Veronika Pavliňáková
Members: Dávid Izsák,
Ivana Chamradová,
Jana Brtníková
Project ID: NU22-08-00454
Masaryk University (main applicant)
Veterinary Research Institute